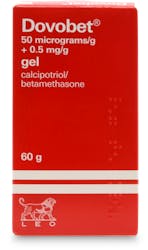
Psoriasis treatment - Dovobet Ointment (PGD) 30g
Psoriasis treatment - Dovobet Ointment (PGD) 30g
What 500,000+ customers say about medino:
Description
Dovobet Ointment (PGD) 30g is a specialised treatment tailored for adults who have stable plaque psoriasis vulgaris. The ointment is formulated with two primary ingredients: calcipotriol, a type of vitamin D analogue that aids in controlling skin cell growth, and betamethasone dipropionate, a corticosteroid that diminishes inflammation. When combined, these components offer a reliable solution for managing the symptoms of psoriasis and enhancing the look of the affected skin.
Key Features
- Tailored for adults with stable plaque psoriasis vulgaris
- Infused with calcipotriol to control skin cell growth
- Contains betamethasone dipropionate for inflammation reduction
- Simple to apply ointment form
- Recommended application once daily for a maximum of 4 weeks
- Clinically tested for safety and efficacy
What symptoms or conditions can be treated with Dovobet Ointment (PGD) 30g?
Dovobet Ointment is primarily formulated to address stable plaque psoriasis vulgaris in adults.
How does Dovobet Ointment (PGD) 30g work?
Dovobet Ointment functions by merging the benefits of calcipotriol, which controls skin cell growth, and betamethasone dipropionate, which diminishes inflammation. This combined approach assists in handling psoriasis symptoms and enhancing the look of the affected skin.
How long does it take for Dovobet Ointment to show results?
While individual outcomes can differ, noticeable improvements in psoriasis symptoms are typically observed within the initial weeks of usage.
Can I use Dovobet Ointment on my face?
No, it's advised not to apply Dovobet Ointment on the face or genital areas since these regions are more susceptible to corticosteroids. For suitable treatment options for these areas, it's best to consult a healthcare professional.
Is Dovobet Ointment suitable for prolonged usage?
Dovobet Ointment is recommended for short durations, not exceeding 4 weeks. For extended treatment needs, it's best to consult a healthcare professional regarding other products more suited for it.
Please note
This treatment is available only after a consultation with our pharmacist via a private service, called PGD (Patient Group Direction).
A picture of the affected area may facilitate and speed up the processing of your order. This can be sent to pharmacy@medino.com before or after you place the order.
It might be necessary to confirm the identity of the intended user through a verification process.
Please read the Patient information leaflet before starting the treatment.
Ingredients
Active ingredients: 1 gram contains 50 micrograms of calcipotriol (as monohydrate) and 0.5 mg of betamethasone (as dipropionate).
Excipient with known effects: Butylhydroxytoluene (E321)
Read the patient leaflet for the full list of ingredients.
Usage and Instructions
Adults: Apply thinly to the affected area once daily for up to 4 weeks. Leave it on the skin all day/night before bathing/showering. Do not use more than 15g daily. Wash your hands before and after use. Avoid sunlight exposure.
Warnings
Dovobet is contraindicated in:
- Under 18 years old
- Pregnancy and breastfeeding
- Erythrodermic, exfoliative and pustular psoriasis
- Calcium metabolism disorders
- Viral (e.g. herpes or varicella) lesions of the skin
- Fungal or bacterial skin infections
- Parasitic infections
- Tuberculosis
- Perioral dermatitis
- Atrophic skin
- Striae atrophicae
- Fragility of skin veins
- Ichthyosis
- Acne vulgaris
- Acne rosacea
- Rosacea
- Ulcers and wounds
Warnings:
Effects on endocrine system: Dovobet gel has a strong steroid; concurrent use with other steroids can lead to systemic side effects due to absorption.
Visual disturbance: Systemic and topical corticosteroids may cause visual issues like blurred vision, cataract, or glaucoma.
Effects on calcium metabolism: Exceeding the recommended dose of Dovobet can result in high calcium levels in the blood, which normalizes after discontinuation.
Local adverse reactions: Dovobet contains a strong steroid that shouldn't be applied on sensitive areas; washing hands post-application prevents accidental transfers.
Concomitant skin infections: If skin infections worsen due to Dovobet, cease corticosteroid treatment.
Discontinuation of treatment: Stopping topical corticosteroid use for psoriasis may have risks; medical oversight is important post-treatment.
Long-term use: Prolonged use increases the risk of adverse reactions; discontinue if side effects appear.
Unevaluated use: Dovobet's effect on guttate psoriasis is unknown.
Concurrent treatment and UV exposure: Limited data on combining Dovobet with other treatments; excessive UV exposure should be limited during Dovobet treatment.
Adverse reactions to excipients: Dovobet gel's excipient can cause skin reactions or eye and mucous membrane irritation.
Avoid contact with textiles that can be easily stained by grease, such as silk
Side Effects
| Category | Frequency | Adverse Effects |
|---|---|---|
| Infections and Infestations | Uncommon (≥1/1,000 to <1/100) | Skin infection* |
| Folliculitis | ||
| Rare (≥1/10,000 to <1/1,000) | Furuncle | |
| Immune System Disorders | Rare (≥1/10,000 to <1/1,000) | Hypersensitivity |
| Metabolism and Nutrition Disorders | Rare (≥1/10,000 to <1/1,000) | Hypercalcaemia |
| Eye Disorders | Not known | Vision, blurred |
| Skin and Subcutaneous Tissue Disorders | Common (≥1/100 to <1/10) | Skin exfoliation |
| Pruritus | ||
| Uncommon (≥1/1,000 to <1/100) | Skin atrophy | |
| Exacerbation of psoriasis | ||
| Dermatitis | ||
| Erythema | ||
| Rash** | ||
| Purpura or ecchymosis | ||
| Skin burning sensation | ||
| Skin irritation | ||
| Rare (≥1/10,000 to <1/1,000) | Pustular psoriasis | |
| Skin striae | ||
| Photosensitivity reaction | ||
| Acne | ||
| Dry skin | ||
| General Disorders and Administration Site Conditions | Uncommon (≥1/1,000 to <1/100) | Application site pigmentation changes |
| Application site pain*** | ||
| Rare (≥1/10,000 to <1/1,000) | Rebound effect | |
*Skin infections including bacterial, fungal, and viral skin infections have been reported.
** Various types of rash reactions such as exfoliative rash, rash papular, and rash pustular have been reported.
*** Application site burning is included in application site pain.
**** Information on the frequency of eye disorders is not known.